Cardiac Rhythm Management Devices Market Trends, Demand and Business Opportunities 2031
Global Cardiac Rhythm Management Devices
Market Overview
The global cardiac
rhythm management devices market was valued at USD 10.8 billion
in 2021 and is projected to grow at a steady CAGR of around 8% over the next
five years. This growth trajectory is supported by the increasing incidence of
cardiovascular diseases worldwide, a rapidly aging population, and continuous
innovation in implantable cardiac devices. Cardiac rhythm management devices
play a crucial role in diagnosing, monitoring, and treating abnormal heart
rhythms, making them indispensable in modern cardiology practices.
Get Free Sample Report: https://meditechinsights.com/cardiac-rhythm-management-devices-market/
Rising awareness about early diagnosis and
timely management of heart rhythm disorders has significantly improved patient
outcomes, further boosting the adoption of these devices across hospitals and
specialty cardiac centers.
The increasing burden of arrhythmias, heart failure, and bradycardia, coupled
with lifestyle-related risk factors such as physical inactivity, obesity, and
stress, continues to expand the addressable patient pool for cardiac rhythm
management solutions.
Understanding Cardiac Rhythm Management
Devices and Their Functions
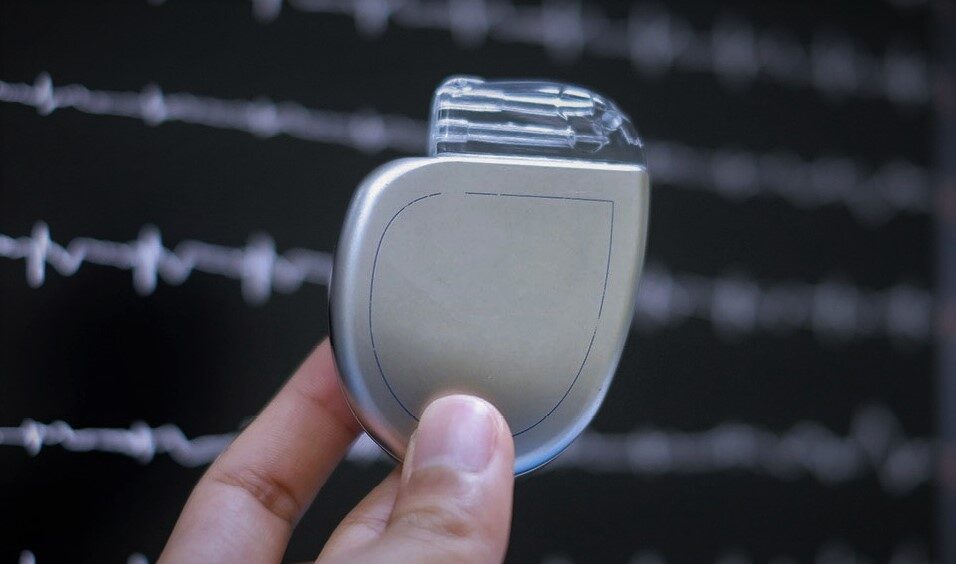
Cardiac rhythm management refers to a medical procedure that involves
implanting a device, typically in the upper chest region, which is connected to
the heart through leads to regulate abnormal heart rhythms using controlled
electrical impulses.
These devices are designed to correct both excessively slow and excessively
fast heartbeats, thereby maintaining a stable and effective cardiac rhythm.
Cardiac rhythm management devices perform several critical clinical functions,
including continuous monitoring of arrhythmias, cardiac resynchronization
therapy for patients with heart failure, pacing support for bradycardia,
defibrillation during life-threatening ventricular arrhythmias, and anti-tachycardia
pacing to terminate abnormal fast rhythms.
The versatility of these devices in addressing a wide spectrum of cardiac
rhythm disorders has made them a cornerstone of advanced cardiac care globally.
Technological Advancements Driving
Market Demand
Rapid technological advancements have transformed cardiac rhythm management
devices into smaller, smarter, and more patient-friendly solutions.
Manufacturers are increasingly focusing on extending battery life to reduce the
need for frequent replacement surgeries, which lowers procedural risks and
long-term healthcare costs.
Miniaturization of devices has significantly improved patient comfort and
reduced complications associated with implantation.
The integration of Bluetooth connectivity enables remote monitoring and data
transmission, allowing physicians to track patient health in real time and
intervene proactively when abnormalities are detected.
MRI-compatible cardiac rhythm management devices have addressed a major
limitation of earlier implants, enabling patients to safely undergo diagnostic
imaging without compromising device functionality.
Such innovations provide manufacturers with a competitive advantage and
encourage continuous investment in research and development to strengthen their
market presence.
Recent Product Approvals and Innovations
in Cardiac Rhythm Management
In May 2022, MicroPort CRM received regulatory approval from China’s National
Medical Products Administration for its Platinium implantable cardioverter
defibrillator, which offers an extended lifespan of approximately 13–14 years,
minimizing replacement-related risks.
In April 2022, Abbott secured U.S. FDA approval for its Aveir single-chamber
leadless pacemaker, designed to treat patients with slow heart rhythms while
eliminating complications associated with traditional leads.
In January 2022, Medtronic obtained approval from Japan’s Ministry of Health,
Labor and Welfare to market and reimburse its Micra AV Transcatheter Pacing
System, recognized as one of the world’s smallest pacemakers.
In July 2020, Abbott gained FDA approval for its Gallant implantable
cardioverter defibrillator and cardiac resynchronization therapy defibrillator
devices, offering MRI compatibility and enhanced battery performance.
In May 2020, Medtronic received FDA approval for its Cobalt and Crome ICD and
CRT-D devices, which combine high power output with advanced connected health
technologies.
In March 2019, Biotronik launched 3 Tesla full-body MR conditional ICD and
CRT-D devices in Europe, noted for their compact size, extended battery life,
and long-term warranty.
Rising Prevalence of Cardiovascular
Diseases Supporting Market Growth
Cardiovascular diseases remain one of the leading causes of mortality
worldwide, accounting for a substantial share of global healthcare burden.
These conditions encompass coronary heart disease, cerebrovascular disease,
rheumatic heart disease, and other disorders affecting the heart and blood
vessels.
Heart attacks and strokes represent the majority of cardiovascular
disease-related deaths, with a significant proportion occurring in individuals
under the age of 70.
Unhealthy lifestyle behaviors such as poor dietary habits, tobacco consumption,
excessive alcohol intake, physical inactivity, and prolonged sedentary routines
significantly increase the risk of developing cardiovascular conditions.
These behavioral risk factors contribute to metabolic abnormalities such as
high blood pressure, elevated blood glucose levels, obesity, and dyslipidemia,
further intensifying the demand for effective cardiac rhythm management
solutions.
Global Statistics Highlighting the
Burden of Cardiovascular Diseases
According to the World Health Organization, cardiovascular diseases account for
approximately 32% of all global deaths, translating to nearly 17.9 million
fatalities each year.
In the United States, the Centers for Disease Control and Prevention reports
that around 659,000 people die annually due to heart disease, making it a
leading cause of death.
Heart disease imposes a significant economic burden in the U.S., costing an
estimated USD 363 billion each year in healthcare services, medications, and
lost productivity.
Coronary heart disease alone was responsible for nearly 360,900 deaths in the
U.S. in 2019, with more than 18.2 million adults living with coronary artery
disease.
Approximately 805,000 individuals experience a heart attack in the U.S. each
year, including both first-time and recurrent events.
In Europe, cardiovascular diseases account for over 3.9 million deaths
annually, with more than 1.8 million occurring within the European Union.
In China, the mortality rate from cardiovascular diseases reached 364.5 deaths
per 100,000 population in 2019, reflecting the growing public health challenge
in emerging economies.
Impact of Aging Population on Market
Expansion
The global geriatric population is increasing at an unprecedented rate,
significantly influencing the demand for cardiac rhythm management devices.
Age-related structural and functional deterioration of the heart increases
susceptibility to arrhythmias, conduction disorders, and heart failure.
Older adults are more likely to require long-term rhythm management solutions,
making implantable cardiac devices a critical component of geriatric
healthcare.
As life expectancy continues to rise, the need for durable, reliable, and
minimally invasive cardiac rhythm management devices is expected to grow
substantially.
Challenges and Limitations Associated
with Implantable CRM Devices
Despite their clinical benefits, implantable cardiac rhythm management devices
face certain challenges that may hinder market growth.
Electrical failures, often caused by insulation breakdown in leads, can result
in device malfunction, inappropriate shocks, or loss of pacing support.
Several product recalls in the past have raised concerns regarding device
reliability and patient safety.
Cybersecurity vulnerabilities pose another challenge, as unauthorized access to
device communication systems could potentially disrupt functionality.
Although the risk of cyberattacks remains low, regulatory bodies and
manufacturers continue to prioritize device security enhancements.
The high cost of implantable cardiac rhythm management devices and associated
surgical procedures may limit adoption in cost-sensitive regions and developing
healthcare systems.
Future Outlook of the Cardiac Rhythm
Management Devices Market
Despite existing challenges, the cardiac rhythm management devices market is
expected to witness robust growth driven by continuous technological
advancements and expanding clinical applications.
Innovations focused on longer battery life, reduced device size, wireless
connectivity, and enhanced safety features are anticipated to improve patient
acceptance and physician confidence.
Growing investments in research, favorable reimbursement policies in developed
regions, and increasing access to advanced cardiac care in emerging markets
will further support market expansion.
Key Players in the Global Cardiac Rhythm
Management Devices Market
- Medtronic plc (Ireland)
- Boston Scientific Corporation (United States)
- Abbott Laboratories (United States)
- BIOTRONIK SE & Co. KG (Germany)
- Stryker Corporation (United States)
- Koninklijke Philips N.V. (Netherlands)
Key Request a free sample copy or view
report summary: https://meditechinsights.com/cardiac-rhythm-management-devices-market/
About Medi-Tech Insights
Medi-Tech Insights is a healthcare-focused
business research & insights firm. Our clients include Fortune 500
companies, blue-chip investors & hyper-growth start-ups. We have completed
100+ projects in Digital Health, Healthcare IT, Medical Technology, Medical
Devices & Pharma Services in the areas of market assessments, due
diligence, competitive intelligence, market sizing and forecasting, pricing
analysis & go-to-market strategy. Our methodology includes rigorous
secondary research combined with deep-dive interviews with industry-leading
CXO, VPs, and key demand/supply side decision-makers.
Comments
Post a Comment